Chemistry: Introduction to Quantitative Chemistry
Table of Contents
View Chemistry Syllabus
Stoichiometry
- Stoichiometry is the branch of chemistry which deals with the calculation of quantities involved in chemical reactions
- This includes the masses and volumes of substances
Patterns in Chemical Reactions
Decomposition Reactions
- Decomposition reactions involve breaking down one compound into 2 or more simpler substances
- Decomposition is an ENDOTHERMIC reaction, meaning it reuqires heat input
- An example of a decomposition reaction is carbonate decomposition:
\(\color{lightgreen}{CuCO_3}\)\(\rightarrow\)\(\color{lightblue}{CuO+CO_2}\)
\(\color{lightgreen}{\text{Green: Reactants}}\)
\(\color{lightblue}{\text{Light Blue: Products}}\)
Decomposition by light
- Some compounds will decompose when exposed to light
- An example is Silver Nitrate (\(AgNO_3\)):
\(\color{lightgreen}{AgNO_3}\)\(\rightarrow\)\(\color{lightblue}{2Ag + 2NO_2 +O_2 }\)
- Light-based decomposition is the basis of film photography
Combustion Reactions
- Combustion reactions occur when something burns
- Combustion reactions are EXOTHERMIC (i.e. light, sound, heat are usually produced)
- Oxygen (or any oxidizer) is always a component of a combustion reaction
- An example of a combustion reaction is burning Propane:
\(\color{lightgreen}{2C_3 H_{8(g)} +7O_{2(g)}}\)$\rightarrow$\(\color{lightblue}{2C_{(s)} + 2CO_{(g)}+ 2CO_{2(g)} +8H_2 O_{(g)}}\)
- Some combustion reactions only have \(CO_2\) and $H_2 O$ as products
- These are known as “complete combustion reactions”
- An example of a complete combustion reaction is burning Methane:
\(\color{lightgreen}{CH_4 +2O_2}\)$\rightarrow$\(\color{lightblue}{CO_2 +2H_2 O}\)
Precipitation Reactions
- When soluble ionic compounds are dissolved in water, the lattice “dissolves”, and the ions are separated
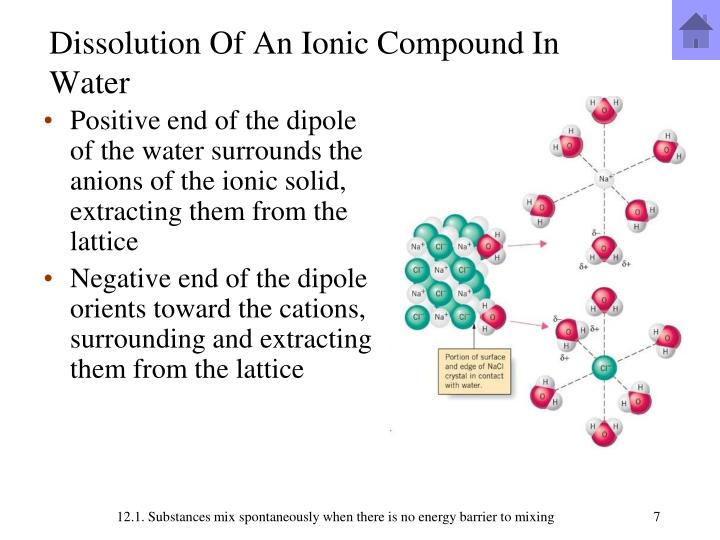
- If two solutions are mixed together, it’s really just 4 different ions suspended in water
- However, certain combinations of ions will form an insoluble compound when mixed
- These compounds will form a PRECIPITATE, a small ionic crystal lattice
- This is known as a precipitation reaction
- An example is mixing sodium sulfide and copper sulfate solutions:
\(\color{lightgreen}{Na_2 S_{(aq)}+CuSO_{4(aq)}}\)\(\rightarrow\)\(\color{lightblue}{CuS_{(s)}+Na_2SO_{4(aq)}}\)
Solubility Rules
The solubility rules are used to determine which compound is the precipitate.
| Ion | Soluble? | Exceptions |
|---|---|---|
| $NO_{3}^-$ | ✅ | ❌ |
| $ClO_{4}^-$ | ✅ | ❌ |
| $Cl^-$ | ✅ | $Ag, Hg_2 , Pb$ |
| $I^-$ | ✅ | $Ag, Hg_2 ,Pb$ |
| $SO_{4}^{2-}$ | ✅ | $Ca, Ba, Sr, Hg, Pb, Ag$ |
| $CO_{3}^{2-}$ | ❌ | Alkalis and Ammonium |
| $PO_4 ^{3-}$ | ❌ | Alkalis and Ammonium |
| $OH^-$ | ❌ | Alkalis, $Ca, Ba, Sr$ |
| $S^{2-}$ | ❌ | Alkalis, Alkaline Earths, Ammonium |
| $Na^+$ | ✅ | ❌ |
| $NH_4 ^+$ | ✅ | ❌ |
| $K^+$ | ✅ | ❌ |
Corrosion Reactions
- Corrosion is a reaction involving a metallic element being converted into a more chemically stable form (e.g. an oxide, hydroxide, or sulfide)
- Combustion and Corrosion are both types of “oxidization reactions”
- Corrosion is EXOTHERMIC, although not as much as combustion
- An example of corrosion is iron rusting:
\(\color{lightgreen}{4Fe+3O_2}\)\(\rightarrow\)\(\color{lightblue}{2Fe_2 O_3}\)
Acids and Bases
Neutralization Reactions
- When an acid and base are added together, they “neutralise” each other
- This creates water and an ionic compound known as a “salt”
- The general formula for acid-base reactions is:
\(\color{lightgreen}{\text{Acid}+\text{Base}}\)\(\rightarrow\)\(\color{lightblue}{\text{Salt}+H_2 O}\)
Acid-Metal Reactions (Displacement Reactions)
- Many metals will react with Acids to produce a “salt” and Hydrogen gas (\(H_2\))
- The general formula for Acid-Metal reactions is:
\(\color{lightgreen}{\text{Acid}+\text{Metal}}\)\(\rightarrow\)\(\color{lightblue}{\text{Salt}+H_2}\)
Acid-Carbonate Reactions
- When an acid reacts with a carbonate compound, the products are always \(CO_2\), \(H_2 O\), and a salt
- The general formula is:
\(\color{lightgreen}{\text{Acid}+\text{Carbonate Compound}}\)\(\rightarrow\)\(\color{lightblue}{\text{Salt}+H_2 O + CO_2 }\)
Conservation of Mass
- Chemical reactions ALWAYS obey the Law of Conservation of Mass:
Matter cannot be created or destroyed, it can only be changed from one form to another.
The Mole Concept
- Chemical formulae simply state the ratio between reactants and products
- For example, \(2HCl + Mg\rightarrow H_2 +MgCl_2\) simply means that for every 2 molecules of $HCl$, there is 1 atom of Magnesium, which produces 1 molecule of Hydrogen gas and 1 molecule of $MgCl_2$ (Magnesium Chloride)
- However, as discussed in Module 1, different particles have different masses
- The Mole concept allows us to easily translate chemical formula ratios into exact masses in units such as grams
Example
- Imagine you had 1 atom of carbon, and 1 of hydrogen
- Notice that carbon is 12 times as massive as hydrogen, despite having the same number of particles
- If you had 1 billion hydrogen atoms, and 1 billion hydrogen atoms, the carbon would weigh 12 times as much as the hydrogen, but have the same number of particles
- Therefore, 12 grams of carbon must have the same number of particles as 1 gram of hydrogen
- If this makes sense, keep going. If not, watch this Youtube video:
The Mole Unit
- The mole is a unit defined as:
The number of Carbon atoms in 12 grams of pure Carbon-12
- This number is known as Avogadro’s Constant, and is represented as \(N_A\)
- This is equal to about \(6.02214076\times 10^{23}\) particles
- 1 Mole is a LOT. For some perspective, Here’s what would happen if you had 1 mole (the unit) of moles (the animal)
- On the periodic table, the atomic weight represents the number of grams per mole of a pure element
- For example, on the periodic table, Hydrogen has an atomic mass of $1.008g/mol$
- Therefore, for every 1.008 grams of pure Hydrogen, there is \(6.02214076\times 10^{23}\) atoms of Hydrogen
Calculating Mole Quantities
\(\color{lightgreen}{n }\color{gray}{=\frac{\color{lightblue}{m}}{\color{pink}{MM}}}=\frac{\color{lightgreen}{N}}{\color{lightgreen}{N_A}}\)
\(\color{lightgreen}{n: \text{Number of moles (mol)}}\)
\(\color{lightblue}{m: \text{mass (g)}}\)
\(\color{pink}{MM: \text{Molecular Mass (g/mol)}}\)
\(\color{lightgreen}{N: \text{Number of particles (No unit)}}\)
\(\color{lightgreen}{N_A : \text{Avogadro’s Constant}(6.022\times 10^{23} mol^{-1})}\)
NOTE: A molecule’s mass is determined by adding up the atomic masses of its composite elements
- For example, $H_2 O$ would have a mass of $H+H+O=1.008+1.008+16=18.016g/mol$
Example Question 1
$\color{cyan}{\text{Calculate the number of moles in 100g of Methane}}$
- Identify the chemical formula of the substance
Methane$=CH_4$
- Find the molecular mass of the substance
$CH_4=12.011+(1.008\cdot 4)=16.033g/mol$
- State the relevant formula
\(n=\frac{m}{MM}\)
- Substitute known values
\(n=\frac{100}{16.033}=6.237mol\)
- Answer the question
\(\text{Therefore 100g of Methane is 6.237mol (3 d.p.)}\)
Example Question 2
$\color{cyan}{\text{Calculate the number of particles in 12 moles of Carbon Dioxide}}$
- Identify the chemical formula of the substance
Carbon Dioxide$=CO_2$
- State the relevant formula
\(n=\frac{N}{N_A}\)
- Rearrange for correct subject
\(N=n\cdot N_A\)
- Substitute known values
\(N=12\cdot 6.022\cdot 10^{23}=7.2264\cdot 10^{24}g\)
- Answer the question
\(\text{Therefore 12mol of Carbon Dioxide is }7.2264\times 10^{24}g\)
Empirical Formulae
- An empirical formula represents the smallest ratio of atoms present in a compound
- For example, Glucose has a molecular formula of \(C_6 H_{12} O_6\), but an empirical formula of \(CH_2 O\)
- Ionic lattices and metallic lattices are ALWAYS represented by an empirical formula e.g. $NaCl$
- Covalent bonds and ionic molecules are represented by a molecular formula e.g. $S_2 Cl_2$
Calculating an empirical formula
- Start with the number of grams of each element
- Divide by the atomic mass
- Find the highest common factor
- Divide the molecular ratio by the highest common factor
Percentage composition
- Percentage composition specifies the percentage by mass of different elements in a compound
Example Question 1
$\color{cyan}{\text{Find the percentage composition by mass for Water} (H_2 O)}$
\(\frac{2\cdot \text{Atomic weight of element}}{\text{Molecular weight of compound}}\cdot 100 = \frac{2\cdot1.008}{18.016}\cdot 100=11.1%\)
The Shortcuts (Molarity and Concentration)
Equations
- \(n=\frac{m}{MM}\)
- \(n=\frac{N}{N_A}\)
- \(n=\frac{v}{M_V}\)
- \(n=c\cdot v\)
$n$ = Number of moles
$m$ = mass (g)
$MM$ = Molar mass (g/mol)
$V$ = Volume (L)
$c$ = Concentration (mol/L)
$M_V$=Molar volume of gases
- $24.79L$ at $1atm$ and \(25^\circ C\){Standard Laboratory Conditions}
- $22.71L$ at $1atm$ and \(0^\circ C\) {Standard Temperature and Pressure})
$N$ = number of particles
\(N_A\) = Avogadro’s constant \((6.022\cdot10^{23})\)
Steps with Worked Example
Sodium and water react to form Sodium Hydroxide and Hydrogen gas. If $12.044\cdot 10^{23}$ atoms of sodium was consumed in the reaction, find the mass of Hydrogen gas formed.
- Balance the Equation and find the mole ratio
\(Na+H_2 O\rightarrow H_2 + NaOH \color{red}{\text{ UNBALANCED 👎}}\) \(2Na+2H_2 O\rightarrow H_2 + 2NaOH \color{green}{\text{ BALANCED 👍}}\) \(\text{Mole ratio= }2:2\rightarrow1:2\)
- Use a formula to calculate the number of moles for the first known
\(n=\frac{N}{N_A}\) \(=\frac{12.044\cdot 10^{23}}{6.022\times 10^{23}}\) \(=2mol\)
- Use the mole ratio determined in step 1 to find the number of moles for the unknown
The ratio of sodium to hydrogen is 2:1
Therefore, for every 2 moles of sodium, there is 1 mole of Hydrogen
- Choose another formula to calculate the unknown (Mass, Volume, Concentration, Number of Particles)
\(n=\frac{m}{MM}\)
\(m=n\cdot MM\)
\(\text{Molar Mass of }H_2 = 1.008\cdot2=2.016 g/mol\)
\(\therefore m=2.016\cdot1=2.016g\)
- Answer the question
\(\text{2.016 grams of Hydrogen gas are formed}\)
Limiting Reagents
- In a chemical equation, a mole ratio is given, which tells you the ideal reaction
- For example, \(2NaI+Pb(NO_3 )_2 \rightarrow PbI_2 + 2NaNO_3\)
- In this formula, the molar ratio is \(2:1\rightarrow 1:2 \)
- So for every 2 moles of $NaI$, 1 mole of $Pb(NO_3 )_2 \) is required
- But what happens if you have more than 1 mole of $Pb(NO_3 )_2 $?
- In this case, after the reaction, there will be some Lead Iodide left over
- The \(NaI\) is in “short supply”, so its mole quantity determines the maximum yield of products which can be formed
- In this case, \(NaI\) is called a Limiting Reagent, because its mole quantity “limits” the amount of product that can be formed
The Gas Laws
Gay-Lussac’s Law of Combining Volumes
“When measured at constant temperature and pressure, the volumes of gases in a chemical reaction show simple, whole number ratios to each other”
- This means that the ratio between the volumes of 2 gases is the same as the ratio between their molar quantities
Avogadro’s Hypothesis
- Yep, Avogadro is back with a hypothesis this time:
Equal volumes of all Gases contain equal numbers of molecules (at the same temperature and pressure)
Molar Volume of Gases
- Avogadro’s Hypothesis has an interesting implication:
- If 1 mole of any chemical contains the same number of particles (Avogadro’s Law)
- If equal volumes of gases contain equal numbers of particles (Avogadro’s Hypothesis)
- Then logically, 1 mole of any gas (at the same temperature and pressure) should have the same volume
- This volume is 24.79L at SLC $ (25^\circ , 100kPa) $, or 22.71L at STP $(0^\circ , 100kPa)$
At other temperatures and pressures, the volume can be calculated using the Ideal Gas Law:
$ \color{red}{P}\color{purple}{V}\color{black}{=}\color{blue}{n}\color{green}{R}\color{orange}{T} $
- $\color{red}\text{P=Pressure (kPa)}$
- $\color{purple}\text{V=Volume (L)}$
- $\color{blue}\text{n=number of Moles (mol)}$
- $\color{orange}\text{T=Temperature (K)}$
- $\color{green}\text{R=Ideal Gas Constant }(N_A \cdot k_B = 8.3145J/K/mol)$
Boyle’s Law
- Boyle’s law describes the relationship between volume and pressure:
If pressure is increased, volume is decreased (as long as temperature is constant) and vice versa
\(\color{cyan}P\propto \frac{1}{V}\)
Charles’ Law
- Charles’ law describes the relationship beteen temperature and pressure
- Fun fact: Gay-Lussac actually made this law, but he got bored of having stuff named after him, and had it named after Charles instead
\(\color{cyan}V\propto T\)
\(\color{cyan}\frac{V}{T}=k\) (where $k$ is a constant)
\(\color{cyan}\frac{V_1}{T_1}=\frac{V_2}{T_2}\)
Gas Laws and the Kelvin Scale
- When Charles and Gay-Lussac made their measurements of gas volumes at different temperatures, they found a trend
- By extrapolating their results, they estimated that at $-273.15 ^\circ C$, the volume of any gas would be zero
- This temperature is known as “Absolute zero,” (0K) as anything colder would have negative volume
- 273.15K = 0$^\circ$C
It was at this point that I realised I’ve spent my whole life spelling Celsius wrong - Jackson Taylor
Common Celsius-Kelvin conversions
| Kelvin | Degrees Celsius |
|---|---|
| 0 | -273.15 |
| 273.15 | 0 |
| 298.15 | 25 |
| 373.15 | 100 |
Gay-Lussac Part 2: Electric Boogaloo
- This guy clearly didn’t have anything better to do, because he ALSO came up with the relationship between pressure and temperature
\(\color{cyan}P\propto T\)
\(\color{cyan}\frac{P}{T}=k\) (where $k$ is a constant)
\(\color{cyan}\frac{P_1}{T_1}=\frac{P_2}{T_2}\)
The Magical Combined Gas Law
- If pressure is inversely proportional to volume (Boyle’s law)
- And Volume relates to temperature (Charles [actually Gay-Lussac but whatever])
- And pressure relates to temperature (Gay-Lussac Part 2)
- Then all the gas laws should be able to be Frankenstein-ed together
\(\color{cyan}\frac{P_1 V_1}{T_1}=\frac{P_2 V_2}{T_2}\)
- Also,
\(\color{cyan}\frac{PV}{T}=nR\) Where R is a constant
- R is known as the “ideal gas constant,” and is on the Chemistry Data Sheet
- The ideal gas constant is Avogadro’s number multiplied by the Boltzmann constant (\(k_B\)), which is the amount of energy (Joules) per degree of temperature (Kelvin or Celcius), and is equal to $1.380649 \times 10^{−23}J/K$
\(\color{cyan}R=N_A\cdot k_B =6.022\times10^{23}\cdot1.380649\times10^{−23}=8.3145J/K/mol\)
Ideal Gas
- The gas laws work perfectly for a so-called “Ideal Gas” which has the following properties:
- The gas particles are so small that they make up an insignificant portion of the volume
- The particles collide in perfect “elastic” collisions i.e. no loss of energy
- There is no attraction between particles
- The closest real gases to the “ideal gas” are Helium, then Hydrogen (as the smallest elements), followed by the rest of the Noble Gases
- The furthest real gases from the ideal gas are \( NH_4 , H_2 O\text{ and }CO_2 \), unless at low pressures and well above their boiling point
| Property | Ideal Gas | Real Gas |
|---|---|---|
| Intermolecular Forces | No intermolecular forces | Particles are attracted by intermolecular forces, especially at high pressure/low temperatures |
| Particle Collisions | No energy is lost in collisions | Some energy is lost in collisions due to the intermolecular forces |
| Particle sizes | Near 0, do not occupy any volume/space | Particles have a very small % of the total space, which increases at higher pressures or low temperatures |
| Obediance to Gas Laws | Always perfectly obeys all gas laws | Obey gas laws at normal temperatures, but not under extreme conditions or close to boiling point |
| Examples | Hydrogen, Helium, Other Noble Gases are the closest to an ideal gas | Oxygen, Nitrogen, Carbon Dioxide, any other gas |
