Chemistry Module 3: Reactive Chemistry
Table of Contents
View Chemistry Syllabus
Types of Reactions
Decomposition Reactions
- Decomposition reactions involve breaking down one compound into 2 or more simpler substances
- Decomposition is an ENDOTHERMIC reaction, meaning it reuqires heat input
- An example of a decomposition reaction is carbonate decomposition:
\(\color{lightgreen}{CuCO_3}\)\(\rightarrow\)\(\color{lightblue}{CuO+CO_2}\)
\(\color{lightgreen}{\text{Green: Reactants}}\)
\(\color{lightblue}{\text{Light Blue: Products}}\)
Decomposition by light
- Some compounds will decompose when exposed to light
- An example is Silver Nitrate (\(AgNO_3\)):
\(\color{lightgreen}{AgNO_3}\)\(\rightarrow\)\(\color{lightblue}{2Ag + 2NO_2 +O_2 }\)
- Light-based decomposition is the basis of film photography
Combustion Reactions
- Combustion reactions occur when something burns
- Combustion reactions are EXOTHERMIC (i.e. light, sound, heat are usually produced)
- Oxygen (or any oxidizer) is always a component of a combustion reaction
- An example of a combustion reaction is burning Propane:
\(\color{lightgreen}{2C_3 H_{8(g)} +7O_{2(g)}}\)$\rightarrow$\(\color{lightblue}{2C_{(s)} + 2CO_{(g)}+ 2CO_{2(g)} +8H_2 O_{(g)}}\)
- Some combustion reactions only have \(CO_2\) and $H_2 O$ as products
- These are known as “complete combustion reactions”
- An example of a complete combustion reaction is burning Methane:
\(\color{lightgreen}{CH_4 +2O_2}\)$\rightarrow$\(\color{lightblue}{CO_2 +2H_2 O}\)
Precipitation Reactions
- When soluble ionic compounds are dissolved in water, the lattice “dissolves”, and the ions are separated
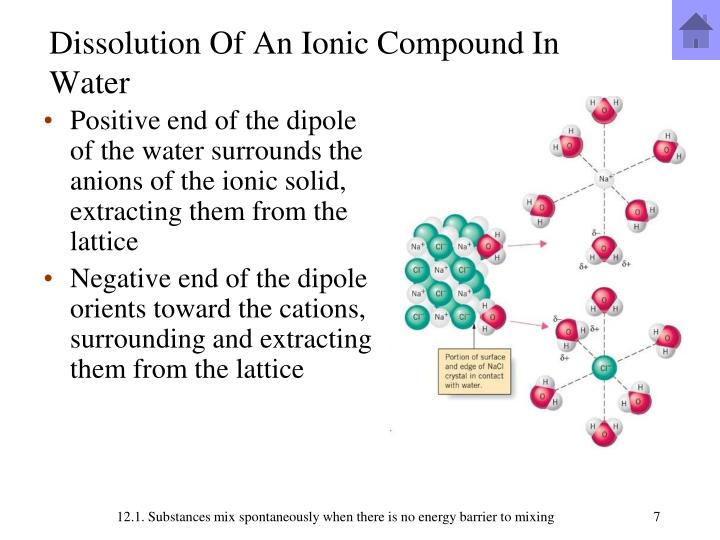
- If two solutions are mixed together, it’s really just 4 different ions suspended in water
- However, certain combinations of ions will form an insoluble compound when mixed
- These compounds will form a PRECIPITATE, a small ionic crystal lattice
- This is known as a precipitation reaction
- An example is mixing sodium sulfide and copper sulfate solutions:
\(\color{lightgreen}{Na_2 S_{(aq)}+CuSO_{4(aq)}}\)\(\rightarrow\)\(\color{lightblue}{CuS_{(s)}+Na_2SO_{4(aq)}}\)
Solubility Rules
The solubility rules are used to determine which compound is the precipitate.
| Ion | Soluble? | Exceptions |
|---|---|---|
| $NO_{3}^-$ | ✅ | ❌ |
| $ClO_{4}^-$ | ✅ | ❌ |
| $Cl^-$ | ✅ | $Ag, Hg_2 , Pb$ |
| $I^-$ | ✅ | $Ag, Hg_2 ,Pb$ |
| $SO_{4}^{2-}$ | ✅ | $Ca, Ba, Sr, Hg, Pb, Ag$ |
| $CO_{3}^{2-}$ | ❌ | Alkalis and Ammonium |
| $PO_4 ^{3-}$ | ❌ | Alkalis and Ammonium |
| $OH^-$ | ❌ | Alkalis, $Ca, Ba, Sr$ |
| $S^{2-}$ | ❌ | Alkalis, Alkaline Earths, Ammonium |
| $Na^+$ | ✅ | ❌ |
| $NH_4 ^+$ | ✅ | ❌ |
| $K^+$ | ✅ | ❌ |
NAGSAG and PMS (Mnemonics)
- NAGSAG can be used to remember common soluble ions:
N - Nitrates ($NO_{3}^-$)
A - Acetates ($C_2 H_3 O_2 ^- $)
G - Group 1 ($Li^+ , Na^+ , K^+ , etc. $)
S - Sulfates ($SO_{4}^{2-}$)
A - Ammonium ($NH_4 ^+$)
G - Group 17 ($F^- , Cl^- , Br^- , I^- , etc.$)
- PMS can be used to remember exceptions:
P - $Pb^{2+}$ (Lead)
M - Mercury
S - Silver
Corrosion Reactions
- Corrosion is a reaction involving a metallic element being converted into a more chemically stable form (e.g. an oxide, hydroxide, or sulfide)
- Combustion and Corrosion are both types of “oxidization reactions”
- Corrosion is EXOTHERMIC, although not as much as combustion
- An example of corrosion is iron rusting:
\(\color{lightgreen}{4Fe+3O_2}\)\(\rightarrow\)\(\color{lightblue}{2Fe_2 O_3}\)
Acids and Bases
Neutralization Reactions
- When an acid and base are added together, they “neutralise” each other
- This creates water and an ionic compound known as a “salt”
- The general formula for acid-base reactions is:
\(\color{lightgreen}{\text{Acid}+\text{Base}}\)\(\rightarrow\)\(\color{lightblue}{\text{Salt}+H_2 O}\)
Acid-Metal Reactions (Displacement Reactions)
- Many metals will react with Acids to produce a “salt” and Hydrogen gas (\(H_2\))
- The general formula for Acid-Metal reactions is:
\(\color{lightgreen}{\text{Acid}+\text{Metal}}\)\(\rightarrow\)\(\color{lightblue}{\text{Salt}+H_2}\)
Acid-Carbonate Reactions
- When an acid reacts with a carbonate compound, the products are always \(\ce{CO2}\), \(H_2 O\), and a salt
- The general formula is:
\(\color{lightgreen}{\text{Acid}+\text{Carbonate Compound}}\)\(\rightarrow\)\(\color{lightblue}{\text{Salt}+H_2 O + CO_2 }\)
Redox Reactions
- Redox is short for “Reduction-Oxidization”
- Redox reactions occur between 2 substances, where electrons are LOST by one (the reductant), and GAINED by the other (the oxidant)
- An easy way to remember this is with AN OIL RIG CAT:
- AN - at the ANode,
- OIL - Oxidization Involves Loss of electrons
- RIG - Reduction Involves Gain of electrons
- CAT - at the CAThode
Rules
- Metals are always reductants, Metal IONS are always Oxidants
- Oxygen has an oxidation state of $2-$ (unless in a peroxide)
- Hydrogen has an oxidation state of 1+ (except in metal hydrides)
- Free elements have an oxidation state of 0
- The oxidation state of an ion is equal to it’s charge
- In compounds, the sum of all oxidation states is 0
- The halogens (F, Cl, Br and I) typically have an oxidation state of 1- in their ionic compounds. In molecular compounds their oxidation number is typically 1- or 7-.
- When naming ionic compounds in which variable oxidation states of metal ions are present, the oxidation state is shown in roman numerals.
$\text{Example: }\ce{FeCl2}$
$\require{color}\text{Iron has an oxidation state of 2+}$
$\text{Therefore, the compound is called Iron } \colorbox{lightgray}{(II)} \text{ Chloride}$
- When Hydrogen ($\ce{H2}$) is burned in Oxygen ($\ce{O2}$), water ($\ce{H2O}$) is formed
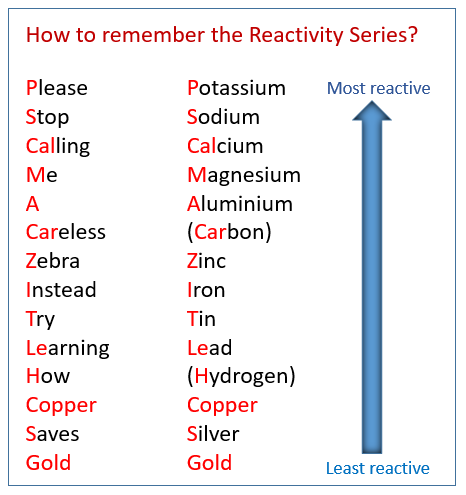
Reactivity Series
Rates of Reaction
Activation Energy $(E_a )$
- Activation energy is the minimum amount of energy required to initiate a reaction
- Activation energy is measured in $kJ/mol$ 1
$\color{green}k=Ae^{\frac{-E_{a}}{RT}}$
$k:\text{Reaction Rate Coefficient}$
$A:\text{Frequency Factor of the reaction}$
$e:\text{Euler’s Number (}e\text{ on calculator, approx. 2.7182)}$
$R:\text{Universal Gas Constant}$
$T:\text{Temperature }(K)$
- According to this equation, the rate of reaction increases with temperature
- However, there are some cases where the activation energy is negative, and so higher temperatures DECREASE the rate of reaction
Catalysis
- Catalysis is the process of increasing the rate of a chemical reaction by introducing a catalyst
- A catalyst is any substance that lowers the activation energy of a reaction WITHOUT MODIFYING THE PRODUCTS
- Catalysts are not consumed by the reaction, and do not change the equilibrium constant of the reaction.
- As a result, catalysts should be written in both the products and reactants of a chemical equation
- Catalysts which trigger a reaction are known as Activators
- The SI unit for Catalysis is the Katal $(Kat)$
$ 1Kat=1mol/s $
- There are 3 main kinds of catalysts:
- Heterogenous Catalysts are those which exist in a different phase from the reaction being catalyzed. For example, solid catalysts the catalyze a reaction in a mixture of liquids and/or gases are heterogeneous catalysts. Surface area is critical to the functioning of this type of catalyst.
- Homogenous Catalysts exist in the same phase as the reactants in the chemical reaction. Organometallic catalysts are one type of homogeneous catalyst.
- Enzymes are protein-based catalysts. They are one type of biocatalyst. Soluble enzymes are homogeneous catalysts, while membrane-bound enzymes are heterogeneous catalysts.